You may need to click on some of the tweets to actually view them properly!
2021-10-04:

Bob’s Med Chem Fact of the Day, Special Weekend Edition:
The last two days: I’ve seen lots of confusion on this site regarding Merck’s new antiviral, Molnupiravir.
The molecule indeed has a checkered past, but let me tell you why I am still interested in its future. ->
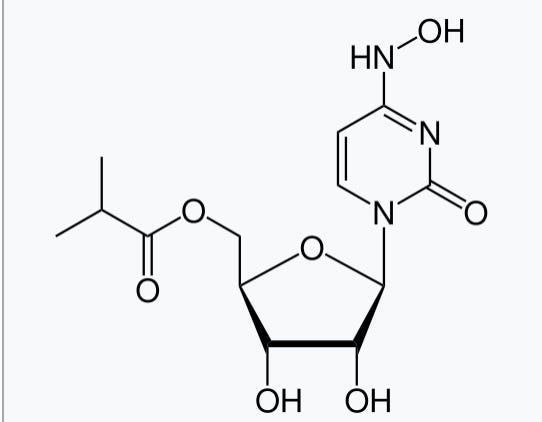

First, let's be clear: N4-hydroxycytidine (which Molnupiravir is a prodrug of) is a bacterial mutagen.
In fact, this is how it was discovered! People knew that hydroxylamine (HA) was mutagenic, and they knew that it was preferentially modifying cytosine to form things like 1.


Thus, researchers thought it would be interesting to synthesize N4-hydroxycytidine directly by reacting cytidine with HA, and testing mutagenicity.
This modified ribonucleoside was indeed highly mutagenic for bacteria.
pubmed.ncbi.nlm.nih.gov/4586551/
pubmed.ncbi.nlm.nih.gov/6160396/


It is not a surprise then that Molnupiravir would show a positive Ames test, which is an *in vitro* test of mutagenicity in bacteria.
As mentioned before, though, a positive AMES test does not always have *in vivo* relevance.

Bob the Grumpy Med Chemist @med_chemist

What a positive AMES test does mean is that you are going to have to do a great deal of work to show that the mutagenic signal is likely not relevant in vivo.
The article below notes some of the other assays done by the Molnupiravir team to show safety


And let’s be clear, it is a lot of work to assess genotoxicity potential in humans. Look at the FDA guidance, if you wish fda.gov/regulatory-inf…
Perhaps most importantly, the human clinical trial data looked good. Also, treatment is short duration, which defrays risk.


Now, let’s look at the main concerns I've seen.
First, this molecule has a past in development. One of the main people raising concerns is actually a well-known Emory professor who wasn’t part of the Emory team now developing the drug (faculty meetings must be fun)


I don’t really find this statement fair.
Emory/Ridgeback/Merck have experienced teams doing the right studies. Men in the trials had to abstain from or use contraception in heterosexual intercourse. We are getting the data to understand the cost/benefit profile of treatment.

Another concern relates to whistleblower complaints from the former head of BARDA. See Wikipedia (en.wikipedia.org/wiki/Molnupira…):


For reference, Bright says his concerns have been mollified (noticeably absent from Wikipedia):
bloomberg.com/news/features/…


And another article I have seen floating around by concerned parties is academic.oup.com/jid/article/22…
With response by Molnupiravir team here

It is likely the developers were well aware of the issues mentioned in the paper. Again, was in vitro, and the team did many follow up in vivo studies.
My grumpy view (not a toxicologist!) is that the UNC study was not gold standard (purity of sample not even mentioned).

The overall point is that, as usual, we have to understand the risks and weigh them with the decent efficacy we have seen in the clinical trials when we consider who this drug is deployed to.
Just note: Much of the world does not have access to mAbs or even shots like USA does.

Couple other things: Molnupiravir (formerly developed as influenza drug) could be very exciting for other applications (flu kills far too many)
Other antiviral development should remain full speed, for many reasons. And you should still get a vaccine, if possible, above all else

Last note:
I have no special knowledge of the development here besides reading what is publicly available, so read it yourself and decide.
@Dereklowe also has some useful overviews of the development and results
science.org/content/blog-p…

2021-10-05:

Bob’s Med Chem Fact of the Day:
In the early days of penicillin, the drug was so scarce that doctors were collecting urine from patients to recover the drug.
The issue was rapid excretion, thus co-dosing with another drug to slow excretion was desired. ->


Probenecid (en.wikipedia.org/wiki/Probenecid) was eventually used to compete for the organic acid transporter, and slow penicillin excretion. Today, it is used in gout to help with uric acid excretion.
Eventually, once penicillin was widely available, large doses alone fixed the issue.

2021-10-06:

Bob's Med Chem Fact of the Day:
There are many reasons to be appreciative of asymmetric organocatalysis today, one of the the Nobel's own cited examples is likely not one of them.
Thalidomide rapidly racemizes in vivo anyway.
doi.org/10.1002/chir.5…
doi.org/10.1016/S0960-…

2021-10-07:

Bob’s Med Chem Fact of the Day:
In Jurassic Park, the “lysine contingency” was a genetic alteration of the dinosaurs such that they could not produce lysine. Thus, park staff would give lysine supplements and the dinosaurs would die outside the park without it.
Not sensible ->


No animals, including us humans, can produce lysine. Thus it is known as an en.wikipedia.org/wiki/Essential…
After escaping, the dinosaurs would simply get their supply of lysine by eating food, like the rest of us.

2021-10-08:

Bob's Med Chem Fact of the Day:
Looking into Ames test (en.wikipedia.org/wiki/Ames_test) after questions about Molnupiravir, one thing I did not fully appreciate was the variability of the strains.
Some strains are even engineered to overproduce enzymes to detect certain mutagens. ->


E.g. some of the Salmonella strains overproduce O-acetyltransferases or nitroreductases to better detect aromatic amine or aromatic nitro mutagenicity.
Interesting overview by scientists in Tobacco industry, who have to worry about the Ames test a lot:
2021-10-12:

Bob’s Med Chem Fact of the Day:
Carbolic acid (also known as en.wikipedia.org/wiki/Phenol) was originally used by Joseph Lister for sterilization of post-operative wounds. The idea came from observing that phenol was used to disinfect sewage. ->


Lister noticed that the waste irrigated fields could be treated with phenol to reduce smell, and animals grazing on the fields would still be fine.
Lister then started treating patients with antiseptic bandages soaked in phenol to reduce infection.

Part of his genius was noticing the differing mortality rates for simple fractures and compound fractures. He reasoned this was because the skin was broken, and thus infection could occur.
He chose to try the antiseptic technique on compound fracture corrections.

This was smart, because he could always later amputate — the standard of care for compound fractures at the time — if things went wrong.
Ultimately, he ended up saving a lot of limbs and lives.


But lest you think chemistry is only an agent for good. Note that phenol injections were the method of choice by the Nazis for the Aktion T4 (en.wikipedia.org/wiki/Aktion_T4) involuntary euthanasia program.
Chemistry is powerful, and your choices in using it can matter.

2021-10-13:

Bob's Med Chem Fact of the Day:
Below is nice example of a compound selectively targeting the site of action (liver). Thus avoids issues of systemic inhibition.
An acid was added to resemble an OATP substrate that should be transported to the liver.

Thanks to @J2_Mousseau and @BagPhos for sharing this review sciencedirect.com/science/articl… where this nice development story was mentioned.

2021-10-14:

Bob’s Med Chem Fact of the Day:
Was reading into fungicides, and the patent story behind succinate dehydrogenase inhibitors (SDHI) is really incredible.
Seems to start in 1985 with patents.google.com/patent/US47420… where Sumitomo patents general structure below


Monsanto then claims in 1991 (patents.google.com/patent/US50933…) difluoromethyl on pyrazole as R1, which was not in Sumitomo patent. Monsanto doesn’t mention optical activity.
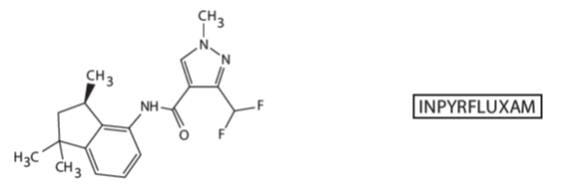
Sumitomo comes back in 2011 and claims mostly R enantiomeric mixture with good fungicidal potency -WO2011162397A1

I believe that mixture would become Sumitomo’s Inpyrfluxam, but Bayer also has patent WO2014083012 on the R enantiomer?
But then Isagoro comes around and puts a fluorine on to patent (WO2012084812), now Fluindapyr
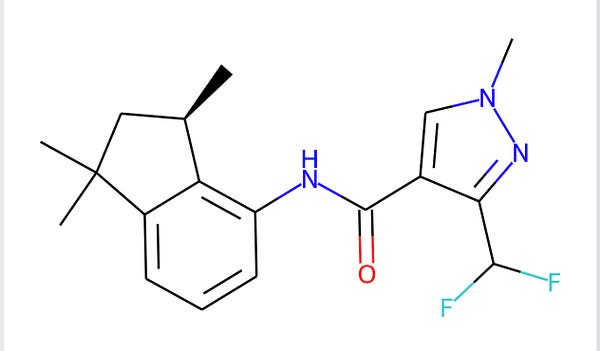


And if you understand all of this, let me know. (I may have some of the history wrong) Different countries have accepted and rejected the various patents. It is quite hard to patent new isomers of a parent compound in India.
The lesson here, I guess, is hire good lawyers.

Much of this info comes from this nice post news.agropages.com/News/NewsDetai… and various wikipedia pages.
2021-10-18:

Bob's Med Chem Fact of the Day:
Botox (Botulinum toxin) is so named because it causes botulism which is so named because it frequently occurred due to poisoning after eating blood sausage. Botulus is latin for sausage.


Thus, celebrity wrinkles can be treated because doctors in the 1800s studied mass botulism events due to sausage poisoning.

2021-10-19:

Bob's Med Chem Fact of the Day:
Goodhart's Law states, "When a measure becomes a target, it ceases to be a good measure."
Just ask past Pharma management about evaluating performance based on # of compounds made. There were indeed lots of compounds made...

Fun fact, Goodhart never said that phrase. The popular formulation of the adage comes from anthropologist Marilyn Strathern

2021-10-20:

Bob's Med Chem Fact of the Day:
On farms, one must be careful about moldy hay because of Sweet Clover disease. If cattle eat moldy hay, they can die of internal bleeding due to fungi converting Coumarin in plants into the anticoagulant Dicoumarol (en.wikipedia.org/wiki/Dicoumarol)
->


During the depression, this became a big issue, because farmers could not afford to discard the bad feed.
Later, one Wisconsin farmer was so sick of losing his prized cattle, that he drove a dead cow and a milk can of unclotted blood to U of Wisconsin, where he met Karl Link. ->

The research on Dicoumarol would eventually lead to the discovery of a related compound that served as a very effective rat poison. That closely related anticoagulant is now known as Warfarin, which you might have heard of.

2021-10-21:

Bob's Med Chem Fact of the Day:
Unlike plasma, urine is unbuffered. Acidity of urine can also be quite different from plasma.
Normal ranges of urine pH:
- human 4.8–7.8
- monkey 5.5–7.4
- rat 7.3–8.5
Interestingly, looks like this can lead to strange toxicity of zwitterions ->

B and C were Gilead compounds that showed strange bladder lesions in monkeys but not rats. Investigations showed this may be likely to the compounds being zwitterionic and detergent-like in low pH of monkey bladder but not higher pH of rat environement.
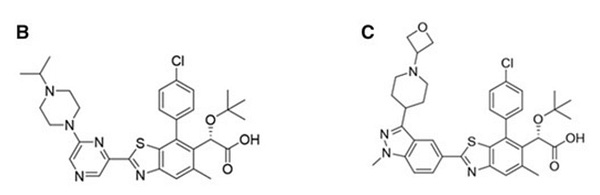

More info here in this interesting tox investigation. Hard to know exactly what is going on in the complicated insides of a monkey, but lesson may be to be careful of compounds that are rigid and highly aromatic, that could be detergent-like.
academic.oup.com/toxsci/article…?
2021-10-22:

Bob's Med Chem Fact of the Day:
Diethylstilbestrol (DES) is a nonsteroidal estrogen once hailed as a wonder drug for women giving birth. Unfortunately, it now carries a legacy of "DES daughters" and "DES sons" who suffered from the issues it caused. Today it is hardly used. ->


DES is also an example of a serendipitous discovery. It tuned out that anol (en.wikipedia.org/wiki/Anol) preparations were causing highly estrogenic activity due to impurities from dimerization.
They were quickly considered a cure for many things from miscarriage to menopause.



We now know it is very dangerous for pregnant women, with many adverse outcomes observed including increased chances of rare vaginal cancers for daughters who were exposed in utero.
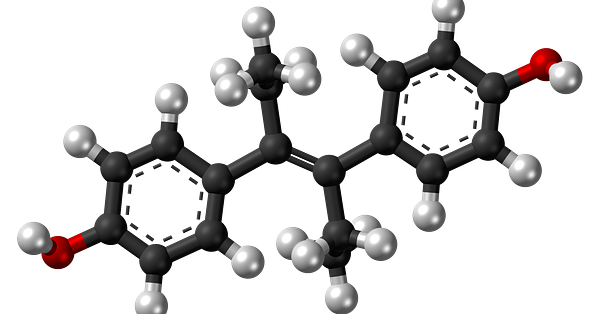

DES was also given to the famous computer scientist and cryptographer Alan Turing (of Enigma fame) as chemical castration treatment as punishment for homosexual acts in 1950s Britain.
en.wikipedia.org/wiki/Alan_Turi… was so named to correct for that shameful past.

2021-10-25:

Bob's Med Chem Fact of the Day:
Polyether antibiotics such as Monensin (en.wikipedia.org/wiki/Monensin) are often used in ruminants. Interestingly, they are ionophores that form complexes with monovalent cations. They can be quite toxic to non-ruminants and equine toxicity is common.


Monensin is often added to cattle feed not only to prevent parasitic Coccidiosis, but also to prevent bloat. Lactic acidosis (en.wikipedia.org/wiki/Lactic_ac…) can be seen in cows if a grain-heavy diet is introduced too quickly. Monensin can help inhibit the lactate-producing bacteria.

2021-10-26:

Bob's Med Chem Fact of the Day:
Labetalol (en.wikipedia.org/wiki/Labetalol) is used to treat high blood pressure. It is given as a diastereoisomeric mixture. The (R,R) isomer alone, Dilevalol, was then developed, but failed in trials due to hepatotoxicity not seen with Labetalol.


I am not sure the mystery of what caused the tox, and why that toxicity would not be seen in the racemic mixture has ever been solved. Some more on the case in this older paper here:
doi.org/10.2165/000020…

2021-10-27:

Bob's Med Chem Fact of the Day:
Suramin (en.wikipedia.org/wiki/Suramin) is an antiprotozoal used to treat African Sleeping Sickness. It was originally Bayer 205, with its structure kept secret as a way to barter for the return of Germany's lost African colonies post World War 1.


Some background: antiprotozoals were of immense interest to the UK and Europe because they could aid colonial expansion in Africa and India. At that time, many people and cattle were dying in those countries.
Thus, the Germans knew the UK was interested in such an agent.

The British government declined and thus there was great interest by non-German scientists in finding the structure that Bayer would not disclose.
Here's a fascinating 1924 doc, where a British scientist discusses the need to develop a similar molecule

The issues it deals with: the need for more organized trial infrastructure, hunting through patents for clues to the structure, uncertainty over IP in countries without established patent systems, sound eerily like many of the discussions we have almost 100 years later.

Another interesting reference parasitesandvectors.biomedcentral.com/articles/10.11…
I'll also note there is a new treatment of sleeping sickness that I wrote about earlier

Bob the Grumpy Med Chemist @med_chemist
2021-10-28:

Bob's Med Chem Fact of the Day:
Many know that Arsenic containing compounds were used as early antiprotozoals and famously Ehrlich's "magic bullet" Salvarsan.
Fewer know that they were still used in poultry production until 2015. E.g. Histostat (en.wikipedia.org/wiki/Nitarsone)


These organoarsenics were used as feed additives to increase growth and prevent parasites.
Compounds such as en.wikipedia.org/wiki/Roxarsone eventually led to very slight increases of inorganic arsenic in chicken livers and meat, which is what led to the discontinuation of the drugs.


@jsrosenblum has reminded me that the inorganic en.wikipedia.org/wiki/Arsenic_t… is still used in humans. My mistake!

2021-10-29:





